In my quest to determine how much more efficient the burning of diesel is compared to gasoline, I could not find one source on the internet that backed up their claims with calculations. For this reason I want to back up my claims on the energy efficiency difference with how exactly the calculations were done.
To determine the energy released from the combustion of diesel and the combustion of gasoline, we must determine the heat of reaction. To do this, we must follow the following steps:
- Gather Info – Such as chemical structure of gasoline and diesel and their associated properties such as density and molecular weight.
- Lay out the chemical equations
- Look up the heat of formation values for each of the components in the chemical reactions.
- Calculate the Heat of Reaction
- Convert to Per Volume Basis
- Determine the energy difference

Gather Info
Chemical Structure of Diesel
The chemical compound of diesel is C12H23. Diesel is really a mixture of hydrocarbons that typically contain between 8 and 21 carbon atoms per molecule.
The density of diesel is about 0.85 kg/L
Chemical Structure of Gasoline
The chemical structure of gasoline is much more straight forward. It is C8H18
The density of gasoline is about 0.75 kg/L.
Chemical Equations
Next step is to lay out the chemical equations we are concerned with:
Combustion of gasoline:
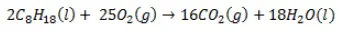
Combustion of diesel:
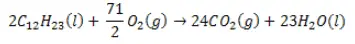
Heat of Formation
The heat of formation is the amount of heat/energy absorbed or evolved when one mole of substance is formed from its constituent elements, each element being in its normal physical state. Definition from Britannica.
The heat of formation has actually been calculated very carefully and tested to provide us with constant values. The values are very easily looked up in a table such as this one. These are clippings of the table with the compound values that I need for the calculation.
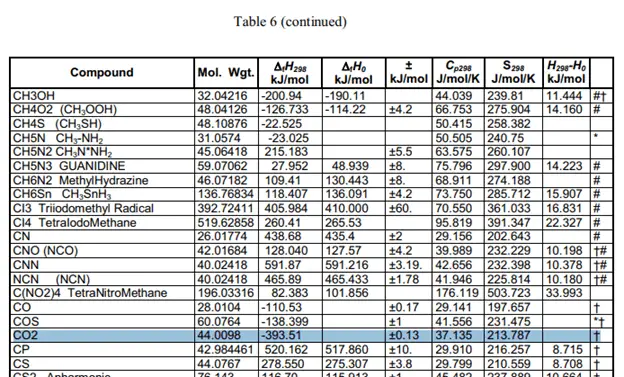
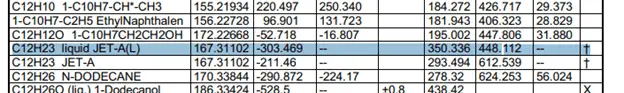
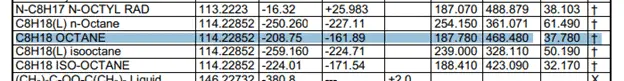
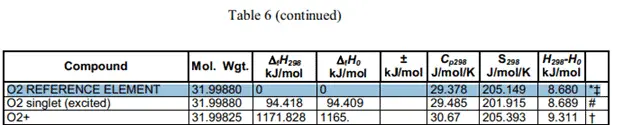
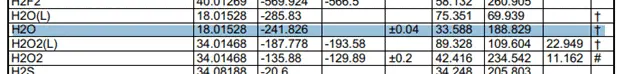
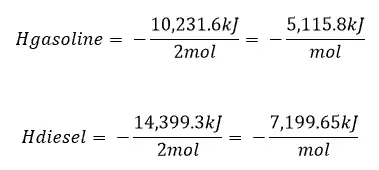
Taking the values of the heat of formation we can find the heat of reaction.
These values were taken from this table.
Enthalpy/ Heat of Reaction
Enthalpy/ is used to calculate the energy released in this chemical reaction. Enthalpy is the total heat content of a system.
The heat of reaction determined at constant pressure is equal to the total heat content of the system and is the amount of heat that must be added or removed during a chemical reaction in order to keep all the substances present at the same temperature. Definition from Britannica.
The heat of reaction is what we need to calculate to determine the amount of energy released during the combustion of diesel and gasoline.
Calculating the heat of reaction indicates whether the reaction is endothermic (if the answer is positive) or exothermic (if the answer is negative) and provides us with the total amount of energy released.
Released in this case since we know that the combustion of fuel is exothermic.
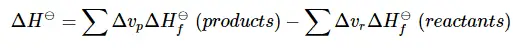
This equation means the sum of the heat of formation of the products – the sum of the heat of formation of the reactants. The v is for the coefficient in front of the chemical compound in the reaction.
Again the chemical reaction equations for reference:
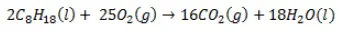
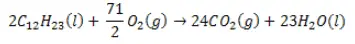
Calculate
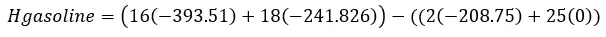
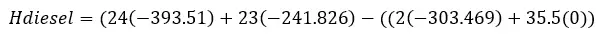
Both the definition for heat of reaction and heat of formation I gathered from Britannica and you can read more on them here and here respectively.
Convert to Per Volume Basis
The densities of diesel and gasoline will be needed here. Since both of these compounds can vary somewhat since they are a mixture of hydrocarbons and we are using a good average, there may be some variation in the density that can be used. For my calculation I am using:
Density of diesel = 0.85kg/L
Density of gasoline = 0.725kg/L
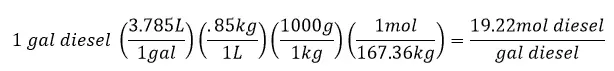
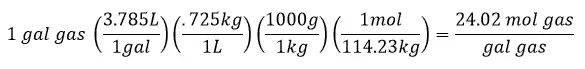
more energy released from the combustion of diesel compared to gasoline by 12.6%
Energy Difference
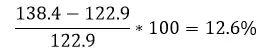
Conclusion
Diesel provides 12.6% more energy per volume than gasoline. This means that by running diesel in your engine rather than gasoline, you should be able to get 12.% more energy out of the same amount of fuel.
This efficiency difference does not account for the difference in engine design between gasoline and diesel engines.
Diesel engines operate at a much higher compression ratio than do gasoline engines and this should greatly increase the engine efficiency as well. To read more on the efficiency of diesel engines compared to gasoline engines read my article: Burning Diesel Fuel: Energy, Efficiency, and Temperature Compared to Gasoline.


